|
Porcine skin is remarkably comparable to human skin. The histological and biochemical properties of porcine skin have been repeatedly shown to be similar to human skin.1-5 The vascular anatomy and collagen fiber arrangement in the dermis, as well as the contents of the stratum corneum glycosphingolipids and ceramides are similar in man and in the domestic pig. Studies examining thickness of various skin layers have shown the stratum corneum thickness in pigs is 21–26 µm which is comparable to human skin.6 The follicular structure of pig skin also resembles that of humans, with hairs and infundibula extending deeply into the dermis. In agreement with published data, pig skin appeared as the most suitable model for human skin.7
Porcine dermis has been used as an in-vitro test substrate to simulate human skin for:
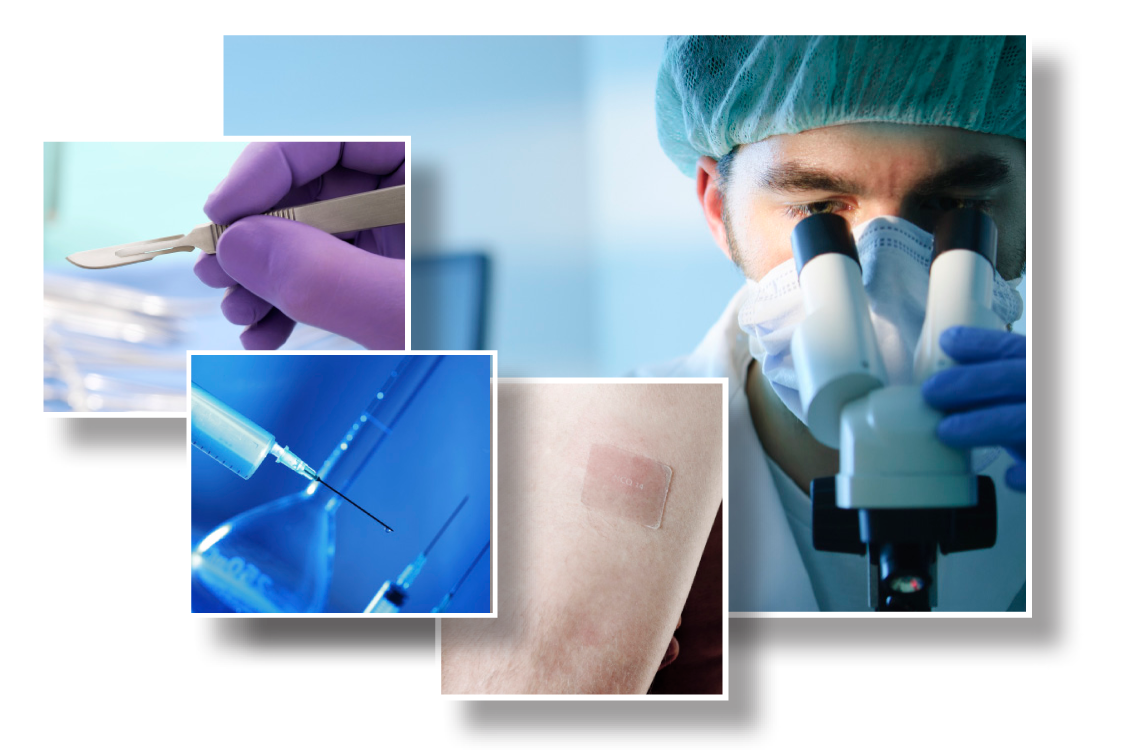
- Hypodermic needle, syringe and cannula sharpness & puncture testing
- Surgical knife/blade sharpness testing
- Transdermal drug delivery & percutaneous absorption/permeation testing
- Soft tissue surgical adhesive & sealant testing
- ASTM 2255-05 Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading
- ASTM F2256-05 Standard Test Method for Strength Properties of Tissue Adhesives in T-Peel by Tension Loading
- ASTM F2458-05 Standard Test Method for Wound Closure Strength of Tissue Adhesives and Sealants
- ASTM F2258-05 Standard Test Method for Strength Properties of Tissue Adhesives in Tension

|
 |
FEATURES & SPECIFICATIONS:
- Non-sterile, frozen porcine skin tissue
- Split thickness (epidermal and dermal surfaces)
- 20cm x 20cm sheets, individually wrapped
- Consistent thickness (1.524mm +/- .254mm)
- Anatomically close to human skin
- Readily available
- Economical
- Stored in standard freezer (less than 14ºF to -13ºF)
- Shaved, white skin only
- Non-perforated
- Includes notch to indicate orientation of the skin
- Widely used in medical device manufacturing for over 20 years
ORDERING INFORMATION:
- Catalog No: I-188
- Visa and MasterCard accepted
- Terms of NET30 available upon credit approval
- Please call for pricing
For more information or to place an order please contact customer service at 1-800-943-4520 or email us at customerservice@stellenmedical.com
|


 1290 Hammond Road • Saint Paul, MN 55110 Ph: 651-426-1496 • T: 800-943-4520 • F: 651-426-3487
1290 Hammond Road • Saint Paul, MN 55110 Ph: 651-426-1496 • T: 800-943-4520 • F: 651-426-3487